The commonly used methods for the determination of ammonia in water are Nessler's reagent colorimetry, phenol-sodium hypochlorite colorimetry, electrode method and titration. Inside And Outside Assembly Flat Press Production Line
GHL company is professional manufacturer for Insulating Glass Production Line, inside and Outside Assembly Flat Press Production Line is one of its categories, to press glass first at outside, then delivery to the pressing part to produce a perfect insulating glass.
GHL company is professional manufacturer for insulating glass production line, main products include all size of inside and outside assembly Ig glazing glass product equipment, online gas filling machine.Insualting glass sealing robot ,butyl sealant coating machine ,spacer bending machine and desiccant filling machine etc.
All products passed CE certificate .
We have many after-sales agencies, could supply perfect after-sales service in the world.
Ig Glass Machine,Hollow Glass Machine,Insulating Glass Machine,Insulating Glass Process Machine Shandong GHL CNC Machinery Co., Ltd , https://www.ghlglassmachine.com
The ammonia nitrogen determination method is mainly selected taking into account the concentration of ammonia and the presence of interfering substances. If turbidity, color, calcium, magnesium, and other hydroxide-precipitable substances are present, they can be removed by pre-distillation. At present, the commonly used distillation apparatus in China is a nitrogen-distillation distillation apparatus with a nitrogen ball, which requires manual distillation, absorption, and titration operations. There are many factors that affect the accuracy of measurement results, and the operation is time-consuming and laborious. Using automatic Kjeldahl apparatus, distillation and titration can be completed automatically. The factors that affect the distillation titration can be automatically controlled, improving the accuracy of the measurement results.
1 Experimental Section 1. 1 Major Instruments and Reagents Kjeldahl: K-370, Buchi, Switzerland; Spectrophotometer: DR 4000/U, Hach USA; Cationic Chromatography: ICS-90, USA Dionex Company; ammonia nitrogen standard stock solution: 1000 mg/L. Shanghai Metrology and Testing Center; sulfuric acid standard working solution: c(1/2 H2SO4)=0.010 30mol/L; boric acid, potassium iodide, mercury dichloride, potassium hydroxide, tetrahydrate potassium sodium tartrate: analytically pure; boric acid absorbing solution: said Take 20 g of boric acid and dissolve it in 1 L of pure water; Nessler's reagent: Weigh 20 g of potassium iodide in about 100 mL of water, add about 10 g of mercury dichloride crystal powder while stirring, until vermilion precipitates. When it is difficult to dissolve, add saturated mercuric chloride solution and stir it well. When trace amounts of vermilion precipitate are not easily dissolved, stop adding mercuric chloride solution; another 60 g of potassium hydroxide is dissolved in water and diluted to 250 After sufficiently cooling to room temperature, the above solution was slowly poured into a potassium hydroxide solution, diluted with water to 400 mL, mixed, and allowed to stand overnight. Take the supernatant solution; Potassium sodium tartrate solution: Dissolve tetrahydrate potassium sodium tartrate 50 g in 100 mL of pure water, heat and boil, let cool, and dilute to 100 mL with purified water; Tetrabutyl ammonium hydroxide solution: Measure 100mL 55% Tetrabutylammonium Hydroxide, diluted with pure water, added to a 2.1L ion chromatography regenerant bottle, add pure water to the bottle neck, shake, add pure water to overflow; Methane sulfonic acid solution: Measure 2.6 mL of methane Sulfonic acid, with ultrapure water to 2 L, as the ion chromatography elution; light magnesium oxide: Shanghai Tongya Chemical Technology Co., Ltd.; experimental water: resistivity greater than 18M8#cm.
1. 2 Experimental method Set the Kjeldahl method parameters, the amount of boric acid absorption solution is 60 mL, and establish the sample table. Add 50 mL of water sample to the distillation tube of Kjeldahl apparatus, add 0.05 g of light magnesium oxide, quickly connect distillation tube to Kjeldahl apparatus, start Kjeldahl apparatus, and start automatic distillation , absorption, titration operation. Prior to the determination of the water sample, a pure water sample was measured in the same manner as the sample was measured, and the amount of titrant consumed in the blank was measured. After the titration is completed, the ammonia nitrogen concentration in the water is automatically calculated.
2 Results and discussion 2.1. Titration endpoint The sample was tested with Kjeldahl apparatus and the endpoint of the titration was determined with a pH electrode. Use the titration data displayed by the instrument to plot the titration curve of ammonia nitrogen, as shown in Figure 1. According to the titration curve, the titration equivalent point can be calculated at pH=4.6~4.8, so the titration end point is set at pH=4.65. 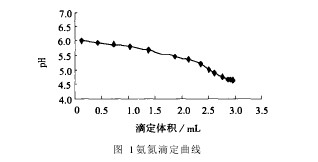
Figure 1 Ammonia Titration Curve 2.2 Magnesium Oxide Dosing The lighter magnesia was added to distill the pre-distilled sample under slightly alkaline conditions. The pH of the water sample has a great influence on the recovery of ammonia nitrogen. If the pH is too high, some organic nitrogen can be converted to ammonia, the pH value is too low, and the recovery of ammonia nitrogen is low [3]. Due to the volume limitation of the distillation tube of the Kjeldahl analyzer, the volume of the water sample was set to 50 mL. The pH of the water sample was adjusted with sodium hydroxide or hydrochloric acid to be neutral. Different masses of magnesium oxide were added to the sample. The pH of the water sample before and after distillation was measured. Tests have shown that it is appropriate to add 0.05 g of magnesium oxide to a 50 mL sample.
2. 3 Steam Power Steam power affects distillation temperature, distillate speed and distillate volume. By changing the steam power, the standard sample with a concentration of 20.0 mg/L was tested. The results showed that steam production in the range of 50% to 100% had no effect on the test results.
2.4 Distillation time Set the steam power to 100%, the reaction time of magnesium oxide to the sample to 5s, change the automatic distillation time (from 120 to 480 s), and test the standard sample with a concentration of 20.0 mg/L. The results showed that a distillation time of 240 s allowed all of the ammonia to be distilled from the sample. As the distillation time increases, the volume of the distilled solution increases rapidly, affecting the accuracy of the measurement results.
2.5 Lower limit of measurement The steam power is set to 100%, the reaction time of magnesium oxide with the sample is 5 s, the distillation time is 240 s, and the ammonia nitrogen standard solution with a concentration of 1.00 mg/L is repeatedly measured four times. The measurement results are 1.1. , 1.1, 1.1, 1.1 mg/L, the relative error of the measurement result is 10%; The ammonia nitrogen standard solution with the concentration of 0.40 mg/L was measured 4 times, the determination result was 0.5, 0.6, 0.5, 0.5 mg/L, respectively. The relative error is 31%, and the lower limit of quantitative determination of the method is 1 mg/L, which is consistent with the blank experimental results.
2. 6 Precision and Accuracy Ammonia nitrogen standard samples and actual water samples were measured five times in succession. The results are shown in Table 1. From Table 1, it can be seen that the measurement results have good precision, and the test values ​​of the standard samples are in agreement with the standard values.
Another two kinds of water samples were added and a certain amount of ammonia nitrogen standard solution was added to carry out the standard recovery test. The results are shown in Table 2. 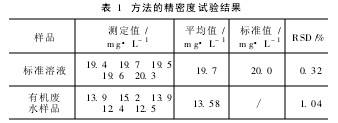
Table 1 Precision test results of the method 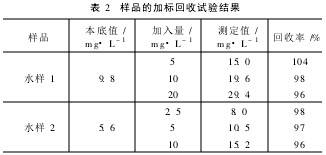
Table 2 sample recovery test results from Table 2 shows that the sample recovery rate of 96% ~ 104%.
2. 7 Method Comparison Ammonia nitrogen in samples was tested by this method, Nessler's reagent colorimetry and ion chromatography, respectively. The results are shown in Table 3. As can be seen from Table 3, for the organic waste water, the determination results using the automatic Kjeldahl determination apparatus and the Nessler reagent colorimetry method are slightly higher than those determined by the ion chromatography method, because part of the organic nitrogen in the organic wastewater will participate in the test under the conditions of the measurement. The reaction, while ion chromatography pretreatment uses C18 cartridges to remove organics, organic nitrogen does not interfere with ion chromatography. 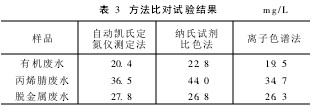
Table 3 Method Comparison Test Results mg/L
3 Conclusions Automatic Kjeldahl meter testing of ammonia in water has the characteristics of automatic simplicity, speed, precision and accuracy of the measurement can meet the analysis needs.