All windshield wiper arms have a hinge at the base that allows the arm to flex as it follows the curvature of the window. If the wiper skips over certain spots, even with a new blade, chances are the hinge is binding from corrosion. It`s most common on rear window wipers since they`re used less often.
You can oil the hinge, but that`s just a temporary fix-the corrosion and binding usually return. To fix the problem permanently, replace the wiper arm (available at an auto parts store).
Now you`re ready to get started!
Plug-In Wiper Arm,Rear Window Wiper Arm ,Rear Windscreen Wiper Arm,Front Wiper Arm COMOTECH ASIA PACIFIC (HANGZHOU)CO.,LTD , https://www.capwiper.com
Is it Time to Change Your Windshield Wiper Arm?
What you`ll need to replace your windshield wiper arm:
Comparative Study on Determination of Protein Content in Feeds by Biuret Method and Kjeldahl Nitrogen Method
The determination of protein content in feedstuffs is usually based on the Kjeldahl method specified by the national standard, but the use of biuret colorimetry to determine the protein content of feedstuffs has also been reported (Chen, 2003). In this experiment, a variety of feed samples using biuret colorimetric method for protein content determination, and the results compared with the Kjeldahl method and analysis, for the practical work to select a reasonable method for the determination of protein content in feed science in accordance with.
1 Biurea method principle
When urea is heated to 150-160 °C, a molecule of ammonia can be removed from two molecules to form biuret (also called biuret). The reaction formula is as follows: 2H2NCONH2→H2NCONHCONH2 + NH3↑ biuret reacts with copper sulfate in alkaline solution to form a purple-red complex, which is called biuret reaction. Compounds containing 2 or more peptide bonds all have a biuret reaction. Because the protein molecule contains multiple peptide bonds (-CO-NH-), it complexes with copper ions in an alkaline solution to form a purple-red complex. Under certain conditions, the color depth is proportional to the protein content. According to this, the protein content can be determined by absorption spectrophotometry, and the maximum absorption wavelength of this complex is 540 nm.
2 experimental materials
2.1 reagent biuret reagent: Weigh CuSO4 · 5H2O 1.5 g, sodium potassium tartrate (NaKC4O6 · 4H2O) 6 g, dissolved in 500 mL of distilled water, add 300 mL of 10% NaOH solution under stirring, and then diluted with distilled water to 1000 mL. With a good storage in plastic bottles. Protein Standard Solution (7 g/100mL), 10% Potassium Hydroxide Solution: Weigh 50g of analytically pure potassium hydroxide in 500 mL of distilled water.
2.2 Main Instrument Crusher; Separation Screen: 0.45 mm (40 mesh); 751 UV/Vis Spectrophotometer; Electric Constant Temperature Water Bath; Analytical Balance: Sensitivity 0.0001 g; Electric Furnace; Glass Beaker; Plugged Glass Test Tube: 25 mL; Volumetric flasks; tray balances, colorimeters, Lovibond colorimeters, etc.
3 Measurement methods
3.1 Standard curve drawing Protein standard solution (7 g/dL) was diluted to 4 mg/mL with 0.1 mol/L NaOH solution. Take 0.0, 0.1, 0.2, 0.3, 0.4, and 0.5 mL of protein standard solution (4 mg/mL) in a 25 mL colorimetric test tube and fill up to 1.0 mL with distilled water. Then add 4.0 mL to each colorimetric tube. The biuret reagent was mixed and incubated at (37±0.2) °C for 30 min. Zero the blank tube, measure the absorbance with a 751 spectrophotometer at a wavelength of 540 nm and a diameter of 1 cm. Take the protein content (mg/mL) as the abscissa, and the absorbance as the ordinate as the standard curve, as shown in Figure 1.
3.2 Determination of protein in feed
3.2.1 Sample Sources There are 10 representative feed samples collected in the feed market.
3.2.2 Sample processing After the sample is crushed, it is filtered through a 40-mesh sieve. Weigh accurately 0.2 g (accurate to 0.0002 g) of feed sample into stoppered test tube, add 10% KOH solution 10 mL, boiling water bath for 30 min, after cooling Distilled water to 25 mL and filtered. Take 0.5 mL of feed filtrate, fill up to 1.0 mL with distilled water, add 4.0 mL biuret reagent, and react at 37±0.2 °C for 30 min. Zero the blank tube and measure the absorbance at 540 nm. Determine the concentration according to the standard curve. The formula is as follows: Feed sample protein content (%) = C × 2 × 25 / W × 1000. In the formula, C is the concentration value (mg/mL) obtained from the standard curve; W is the weight of the feed sample (g).
4 Results and Analysis
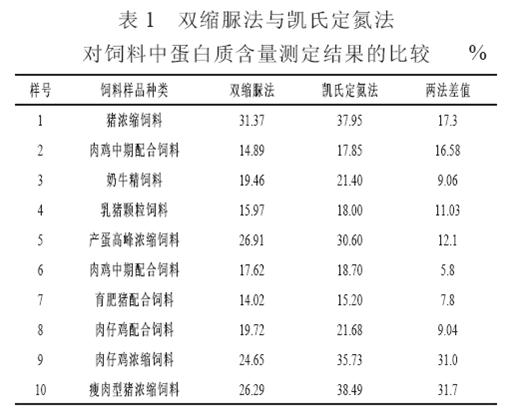
4.1 Comparison results of different feed samples are shown in Table 1. From Table 1, it can be seen that the results of the biuret method on the protein content of feed and the Kjeldahl assay result are quite different, which is 15.1% (5.8% to 31.7 %) lower than the Kjeldahl method. . Sun Jianping and Hou Caiyun (2005) believe that the bis-urea assay results are 10% to 20% smaller than the Kjeldahl method. The protein content measured by the Kjeldahl method is crude protein, which is the nitrogen content multiplied by 6.25, which includes inorganic nitrogen and amino acids. The protein content measured by the biuret method is a true protein. It is the protein that the alkaline solution can dissolve, and it reacts with the biuret to become the coloring part of the complex; The reaction of the insoluble part of the protein and the biuret reagent is little, even if there is a reaction, the resulting colored network The compound was filtered and was not present in the final colorimetric solution. Therefore, the absorbance value read out in the colorimetric assay is simply the absorbance value of the colored complex formed by the soluble protein. In addition, the protein material added to the feed is more complex, including soybean meal, peanut meal, cottonseed cake, rapeseed cake, fish meal, and feather meal. Although crushed, sieved, and boiled in the lye, some proteins are still insoluble in the test liquid, causing a greater error than the actual value. In addition, a large amount of crude fiber is contained in the feed. For samples with a high crude fiber content, the results of the biuret method are not very stable.
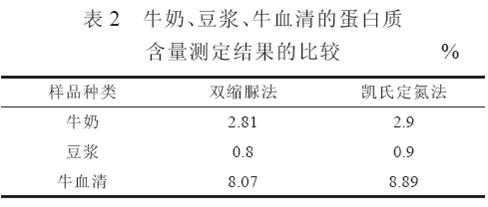
4.2 The results of comparative experiments in milk, soymilk and bovine serum are shown in Table 2. It can be seen from Table 2 that the use of the biuret method for determining the protein content of milk, soybean milk, and bovine serum is similar to the Kjeldahl method because the proteins contained in these samples are highly soluble and contain relatively few protein types. The nature is close to the standard protein solution.
4.3 Determination of Protein Content in Feed by Biuret Method Precision Experiments For feed samples No. 5 and No. 6, six samples were taken and the protein content was determined by biuret method. The results are shown in Table 3. From Table 3, it can be seen that the results of the parallel determination of the same feed sample by the biuret method have better reproducibility and higher precision. In accordance with the relative deviation range of the protein determination results specified in the national standard, the average value is significantly different from the Kjeldahl results.
5 Summary
The biuret method for measuring feed protein, although not digested, does not produce harmful gases, but it does not save time. Starch in feed is gelatinized by alkaline solution and heat treatment, difficult to filter, especially with high content of starch, can only filter 1-2 mL at 2 h (concentrate is relatively fast), and this method is subject to the working environment (temperature, etc.) The impact is greater, and it will be more time consuming if each time you make a standard curve. The components in the feed are complex, and some ions have an effect on the color reaction, which is also the cause of unstable results.
In summary, biuret method is not suitable for the determination of protein content in compound feeds and concentrated feeds.